The Covid-19 with its global spread has impacted heavily on the multilateral institutions as never before. The closing of national boundaries and sudden disruption of global network of transport and supply chain has led to skepticism about the capacity of multilateralism and multilateral institutions. But the very transnational implications of the pandemic and multilateral cooperation in responses, sharing of pandemic information and research cooperation signifies that world needs the multilateral cooperation and enhanced institutional capacity more than before to deal with impending pandemic which are going to recur more frequently as per the medical research and predictions.
COVID-19 and Its Impact warrants Multilateral Response
COVID-19 had massively disrupted health systems and health service delivery in low- and middle-income countries in Africa and Asia in 2020.
COVID-19 has gulped the entire globe and every single individual is fighting against this pandemic either emotionally or through financial setbacks. The medical system has collapsed in almost all the countries including the most developed and rich countries like the USA, the UK and most of the EU countries. Since this is an infectious disease, it is very challenging for India to control the spread but also to provide the healthcare facility to the patient who can’t afford it, specifically in rural areas where healthcare facilities are not so advanced.
India is the pharmacy hub and is recognized to be a major medicine manufacturer and exporter across the globe.
During the pandemic, the demand for medicine is increased which was not projected. The medicines that we believe was for worms and for the ARV treatments are found to be effective for COVID- 19 treatment.
Due to the sudden spurt in the demand for medicines and medical devices associated with it (e,g. Oxygen cylinders, ventilators etc.) across the globe, the countries that follow stringent regulatory guidelines for importation of the drugs, has also relaxed the registration/importation requirement. In countries like Brazil, Columbia, Chile, Uzbekistan where registration is mandatory, the Ministry of Health of these countries has put mild restrictions or allow to import the of goods without registration or with the import waiver. This turned to be an immediate opportunity for a pharmacy hub like India, where all the small manufacturers could supply the goods to these countries.
This pandemic has disrupted the Healthcare system across the globe. It also brought an opportunity to many pharma companies to supply emergency medicine to patients suffering from COVID-19. This pandemic, to a certain extent, opened the barriers for free trade medicine.
India (Source: Global Fund)
Countries across South Asia experienced a deadly surge of COVID-19. The region, home to almost 2 billion people, now accounts for half of all new, known infections globally. Every second, more than three new cases are recorded.
Hospitals are overwhelmed, there is an acute lack of oxygen and other life-saving medical supplies, and there is a real risk of fragile health systems collapsing. In the countries that are being hardest hit, entire families are losing their lives to the virus.
The sheer speed and scale of infection is pushing health systems in India, Nepal, Sri Lanka and the Maldives to the brink – with fears that Bangladesh, Pakistan, Bhutan and Afghanistan could face similar devastating surges. The speed of the virus is outstripping countries’ ability to treat patients and save lives.
The Global Fund has approved US$75 million in fast-track funding to support India’s response to the COVID-19 crisis that is devastating the country. This new funding will support India in purchasing oxygen concentrators and Pressure Swing Adsorption (PSA) oxygen plants to help meet the medium-term needs for medical oxygen.
Alongside corticosteroids, medical oxygen is the only proven lifesaving treatment for the most seriously ill COVID-19 patients. Without oxygen, COVID-19 patients suffering from hypoxaemia – an abnormally low level of oxygen in the blood – will likely die.
The most immediate gap is medical oxygen, we also need to invest in the health systems and health workers needed to treat the patients and respond to COVID-19. Oxygen will save lives, but it is only part of the solution. Need to massively scale up testing and vaccinations to stop the spread of COVID-19 in India and worldwide.
The new funding is in addition to US$36.8 million approved for India in 2020 through the Global Fund’s COVID-19 Response Mechanism to help mitigate the pandemic’s impact on HIV, TB and malaria programs, purchase personal protective equipment (PPE) for front-line health workers, procure testing equipment and strengthen health systems – in particular laboratory capacities and community health networks.
Even before the pandemic, low- and middle-income countries were facing chronic oxygen shortages for treating other conditions and diseases, and COVID-19 is now pushing health systems to the brink. As India’s acute oxygen needs are being addressed by its government and partners, the Global Fund’s response is supporting higher output oxygen solutions that will be beneficial as the COVID-19 response develops.
Indian Government has taken forward steps to be independent in Oxygen production and has initiated setting up the plant to produce Oxygen to mitigate the risk of Oxygen shortage and minimize the risk of external dependency. Even in Maharashtra, major Sugar Plants started producing oxygen so that to face the shortage of oxygen during the 3rd wave if any.
India experienced a deadly shortage of Medicines… specifically Remdesivir and Tocizulimab. Remdesivir is patented by Gilead, it is out license to the few companies in India to manufacture and to name a few is Cipla, Zydus, Biocon, Hetero, Mylan. Despite having multiple manufacturers, there was a major shortage of this product during the second wave as no one has expected the spread of the virus so fast.
What happened in India during the second wave, can happen elsewhere. It’s a warning that we cannot let our guard down. The emergence and rapid spread of more virulent variants highlight the importance of a global and comprehensive approach – including testing, treatments such as corticosteroids and medical oxygen, and vaccines – to fight this pandemic. No country is safe until we have COVID-19 under control everywhere.
Medicine shortages across the globe
Drug shortage is a global issue affecting low, middle, and high-income countries. Many countries have developed various strategies to overcome the problem, while the problem is accelerating, affecting the whole world. All types of drugs, such as essential life-saving drugs, oncology medicines, antimicrobial drugs, analgesics, opioids, cardiovascular drugs, radiopharmaceutical, and parenteral products, are liable to the shortage. Among all pharmaceutical dosage forms, sterile injectable products have a higher risk of shortage than other forms. The causes of shortage are multifactorial, including supply issues, demand issues, and regulatory issues. Supply issues consist of manufacturing problems, unavailability of raw materials, logistic problems, and business problems. In contrast, demand issues include just-in-time inventory, higher demand for a product, seasonal demand, and unpredictable demand. For regulatory issues, one important factor is the lack of marketing authorization in the country to cater for the unmet demand. .
In line with us, few market entry opportunities in semi-regulated countries.
Uzbekistan
The importation of medicine in Uzbekistan without registration is not permissible. Due to the bad pandemic situation Uzbekistan government, issued a special Decree, according to it importers can supply medicine without registration at the Ministry of Health’s Pharma Committee. Though the importation of the medicine was time-bounded, it was a gateway for the small pharma manufacturing holding WHO GMP certification, to supply the goods without registration and with import waiver.
In this regard, the product imported during this period was
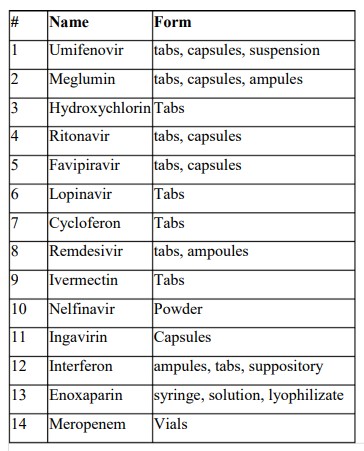
Though this was the one time supply to meet the unmet demand, the supplier had received a certificate that can be leveraged while submitting the dossier for registration.
(Source: email received from Uzbekistan customer for the supply of medicine)
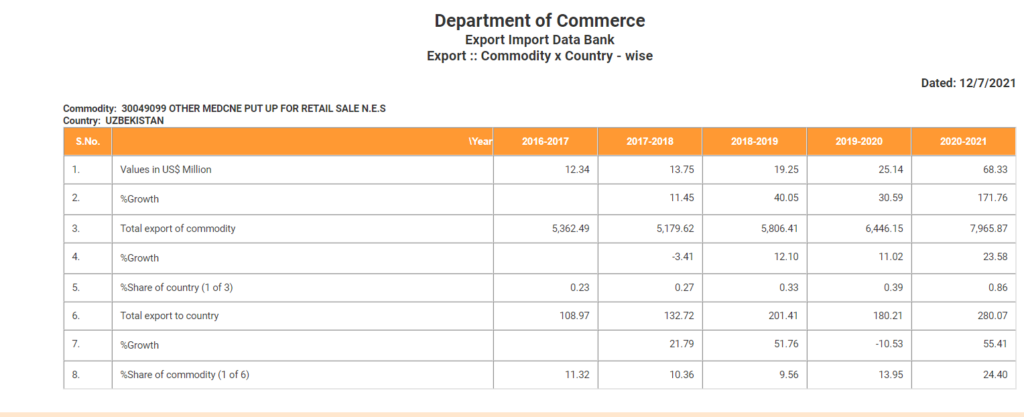
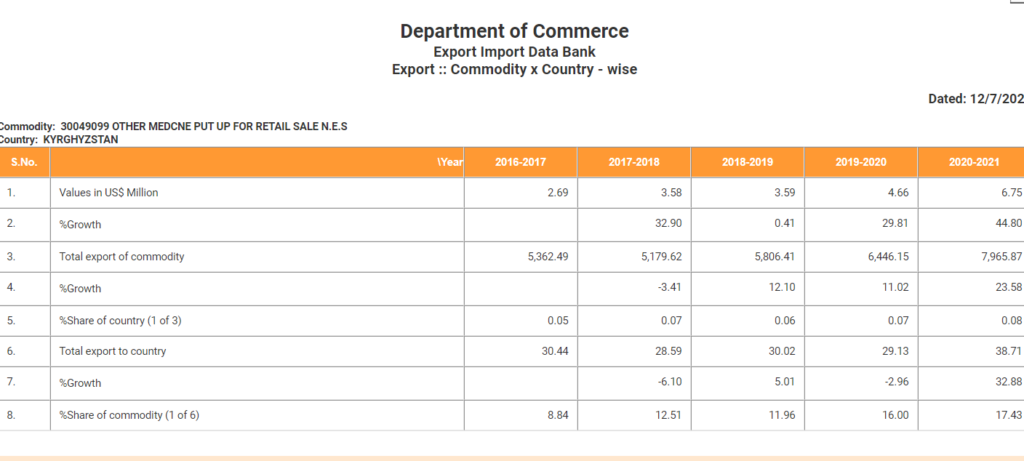
The table mentioned above indicates the growth in the export of Meropenem by 44.8% in the FY 2020-21 which is drastically high compared to previous financial year.
(Source: Department of Commerce website)
Kyrgyzstan
Medicines imported and / or produced by pharmaceutical organizations that have a certificate of compliance with Good Manufacturing Practice or Good Distribution Practice with a certificate of Good Manufacturing Practice for products recognized in the Kyrgyz Republic are not subject to quality assessment.
The procedure for assessing the quality of medicines, the criteria for exemption from serial control and the amount of the fee are determined by the Government of the Kyrgyz Republic.
During the pandemic, the Government of the Kyrgyz Republic allowed the importation of pharmaceutical drugs without any registration. As per the MOH registration guidelines, the timeline to register the product is appx. 6-9 months. But due to relaxation in importation of the medicines in the country, all the small and medium pharma companies were benefited. The major chunk of the pharma product supplied by Indian pharma companies
The Ministry coordinates the work on supply of the population and public health care organizations with medicines and organizes interaction with the domestic pharmaceutical industry in the field of production and supply of medicines to the public and public health care organizations, in forming the Essential Drug List. Ministry also provides control over the rational use of medicines, regardless of the source of procurement by health care organizations. Considering these parameters, MOH allowed importers to procure medicines considered as Essential medicine during the Pandemic without registration or with minimal documentation.
(Source: Emails from Kyrgyzstan customers for the requirement of essential medicines)
Kazakhstan
Kazakhstan’s Pharma Industry Has Big Plans To Expand The Market
Kazakhstan’s pharmaceutical market showed positive dynamics in 2020. In the first 9 months of last year, market size for finished pharmaceutical products grew to KZT460bn ($1bn), up 22% year-on-year.
Other areas of the Kazakhstan pharmaceutical market have also shown high growth increases: the volume of purchases for outpatient drug provision over the past year increased by 41%, and purchases for hospitals grew by 30%. In monetary terms, these areas accounted for KZT115bn and KZT91bn, respectively.
The key reason was sharp increase in demand for medicines because of ripple effects from the global coronavirus pandemic. In the first quarter, the volume of purchases from medical institutions and pharmacies increased. This was caused by preparations for the first wave of COVID-19. The next factors were the rising cost of drugs and the emergence of new treatments. Due to the pandemic, many new drugs have appeared on the market that have helped in the prevention and control of the consequences of COVID-19. The rapid introduction of new treatments, some with limited supplies, has driven up the cost of drugs.
(Source : Pharmaintelligence – Informa)
Most of the market is import-dependent. The product volume produced by domestic manufacturers totalled KZT42bn in 2018. At present, the share of domestic manufacturers in the procurement of medicines and medical devices has grown to 30% and continues to grow steadily
Kazakhstan is also committed to improving the quality standards under the interstate documents, which were adopted to ensure the launch of common market of the EEU. These documents define the general requirements for drug production, storage and sale, which meet the highest quality standard (GMP, GCP, GDP, GLPP etc).
All Pharmaceuticals, Product of Medicinal use and medical equipment with active registration in the Republic of Kazakhstan can be found at the State Register of the National Center for Medicines and Medical Devices.
The Ministry of Health has allowed importation of the medicine without registration during the pandemic COVID-19. This relaxation was valid till December 2020 and it triggers many Indian pharma company to supply finished dosage form to the country without registration which otherwise impossible to export without registration.
LATAM Pharma Industry
Chile, Columbia, Mexico Brazil, these are the most advanced countries and follows stringent regulatory guidelines. Despite this, the Ministry of Health of these respective countries allowed to import the drugs without registration and without GMP approval. GMP approval is one of the biggest criteria specifically to supply the medicine to Mexico and Brazil. Cofepris (Mexico) and ANVISA (Brazil) are considered as one of the Stringent Regulatory Authorities (SRA). To register the product in these countries, the manufacturing factory should hold the EU or other SRA approval. Hence supply or registration of the product in these countries is a costly affair.
Due to the surge in COVID-19 patients, the MOH of these countries allowed local distributors to import the medicine from other manufacturers without EU GMP accreditation. This was benefitted by Indian pharma companies who hold WHO GMP certification for the manufacturing units producing medicines like, cough syrup, injections, tablets.
Brazil
Anvisa extended the validity of the Board of Directors (RDCs) 483/2021 and 484/2021, which provide for exceptional and temporary measures to emergency relax the sanitary requirements related to register and importation of medicines and essential products to face Covid-19.
The Resolution of the Collegiate Board (RDC) 484/2021 was extended until December 31, 2021. This is the temporary and extraordinary procedure for the authorization, on an emergency basis, of drugs used for intubation of patients with Covid-19. The measure applies to anaesthetics, sedatives, neuromuscular blockers and other hospital medications used to maintain the lives of patients.
The marketing authorization of such products shall be carried out by means of notification. The notification allows products to be immediately manufactured and readily made available to hospitals and clinics throughout Brazil.
The Resolution of the Collegiate Board (RDC) 483/2021 was extended until September 14, 2021. This DRC allows the direct importation of a list of medicines and medical devices not regularized in the country, on an exceptional and temporary basis.
It also includes the product list including antibiotics (polymyxin Band sulfamethoxazole/trimethoprim).
(Source: www.gov.br)
Chile
When Covid-19 cases have been on the rise and patients in the ICU have remained above 3 thousand cases per day, Cenabast’s work becomes fundamental to the work of each of the teams that assist the victims of this pandemic to keep them alive.
That’s why the procurement of essential drugs for intubated patients, such as Atracurius, Fentanyl and Midazolam, has been a priority for Cenabast throughout this pandemic period.
During the year 2020, Cenabast made purchases of drugs for Covid19 patients, for an amount of $ 17,346 million pesos, which also involved the intermediation for private clinics, due to the consideration of the process of centralization of supply carried out by the Ministry of Health, for the entire system, both public and private with 13,000,660 unit doses in total.
In this period, there were some failed purchasing processes, with suppliers that presented discontinuity in delivery, which forced the health institution to buy from other suppliers at high prices, as well as to import finished products or obtain raw materials, for the production of medicines in the country. Centabast and MOH allowed importing the medicines without registration and with import waivers.
Despite all the drawbacks mentioned, Cenabast kept at all times available stocks of products necessary for the entire Health Network.
Cenabast’s (in coordination with MOH) work is fundamental, and the timely supply gives the healthcare system security to work because otherwise, a drug with a different profile would have to use, which may not be the most appropriate for some patients.
The distribution in Chile has been directed by the Ministry of Health, in coordination with Cenabast. This is operating 24/7 from the logistics operator.
This 2021, Cenabast has managed to maintain the supply of the aforementioned products, even with the rise of unexpected infections. This, despite the high prices that have been recorded, as a result of quarantines and increased infections in the countries of origin of the drugs, as well as the growth in costs associated with international trade operations. Thus, the provision of ICU drugs for the first quarter of 2021 was as follows:
ICU Drug Acquisition as of April 2021
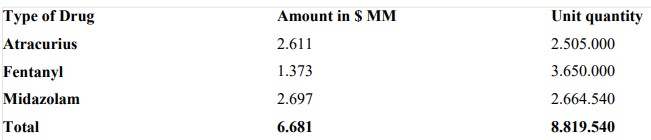
India’s exports data reflect sudden spurts in the supply of drugs like Midazolam and Fentanyl in 2020-21. This was due to the chaotic situation in the country due to the lack of medicines and waiver by MOH to allow the import without registration.
(Source: Website:- Cenabast tender procurement, CENABAST CHILE.)
Conclusion:
These are the countries in which the Ministry of Health of the respective countries allowed to import the drugs with a minimum regulatory requirement which benefitted Indian pharmaceutical companies/sector to grow.

